Colligative properties worksheet answer key – Welcome to the realm of colligative properties, where the concentration of solute particles dictates the behavior of solutions. Our comprehensive worksheet answer key serves as your guide, unraveling the mysteries of vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
Prepare to immerse yourself in a world of scientific discovery and practical applications.
Colligative properties hold immense significance in various fields, from chemistry and biology to engineering and medicine. By understanding these properties, scientists can determine the molar mass of solutes, predict the freezing and boiling points of solutions, and even harness osmotic pressure for water purification and desalination.
Join us as we delve into the fascinating world of colligative properties, empowering you with the knowledge to tackle complex problems and make informed decisions.
Colligative Properties
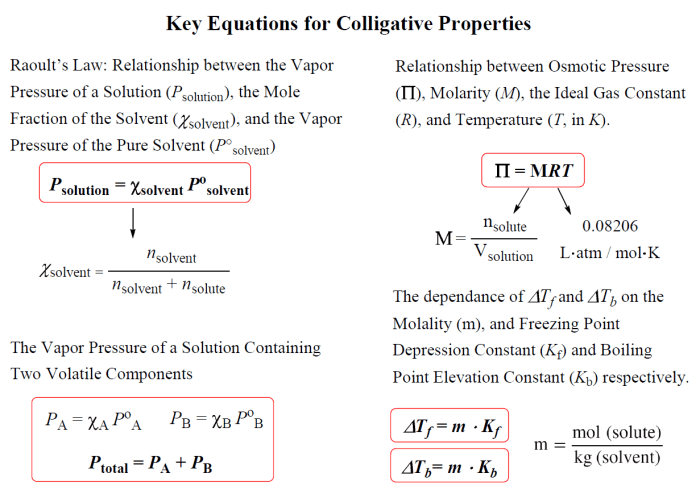
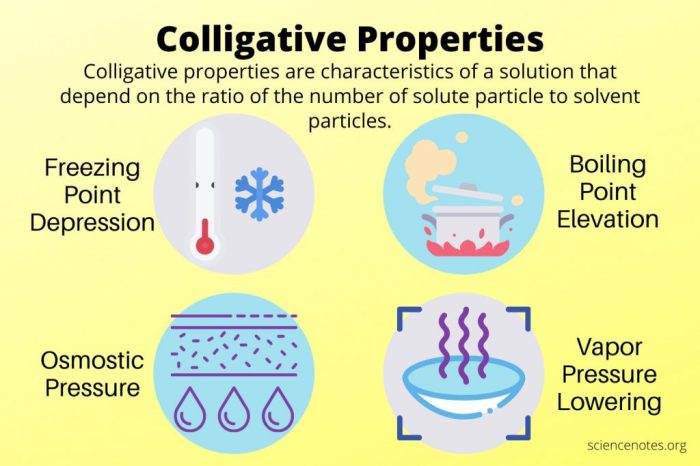
Colligative properties are properties of solutions that depend on the concentration of solute particles, but not on the identity of the solute. The four main types of colligative properties are vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
Types of Colligative Properties
- Vapor pressure loweringis the decrease in the vapor pressure of a solvent when a non-volatile solute is added to it.
- Boiling point elevationis the increase in the boiling point of a solvent when a non-volatile solute is added to it.
- Freezing point depressionis the decrease in the freezing point of a solvent when a non-volatile solute is added to it.
- Osmotic pressureis the pressure that must be applied to a solution to prevent the passage of solvent molecules from a pure solvent into the solution.
Applications of Colligative Properties, Colligative properties worksheet answer key
- Colligative properties can be used to determine the molar mass of a solute.
- Colligative properties can be used to measure the freezing point of a solution.
- Colligative properties can be used to predict the boiling point of a solution.
FAQ: Colligative Properties Worksheet Answer Key
What is the significance of colligative properties?
Colligative properties provide crucial information about the concentration of solute particles in a solution, enabling scientists to determine molar mass, predict boiling and freezing points, and understand osmotic pressure.
How are colligative properties used in real-world applications?
Colligative properties find applications in diverse fields, including determining the molar mass of unknown substances, predicting the freezing point of antifreeze, and designing desalination plants.
Can colligative properties be used to distinguish between different solutes?
Yes, colligative properties depend solely on the concentration of solute particles, regardless of their identity. This makes them useful for distinguishing between different solutes with the same concentration.