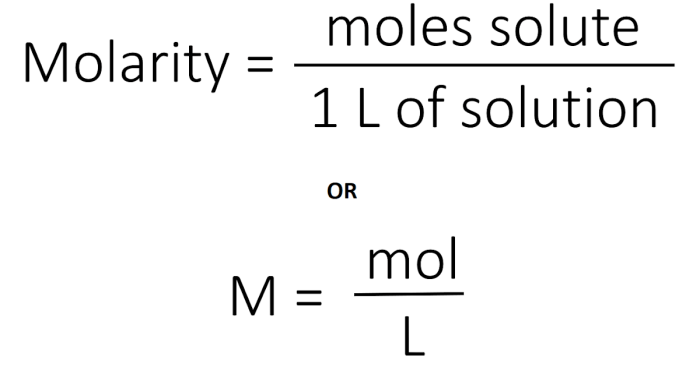Delving into the realm of chemistry, concentrations worksheet molarity and molality unveils the intricacies of solution concentrations. This in-depth guide explores the fundamental concepts, calculations, and applications of these two essential measures, empowering you with a comprehensive understanding of chemical solutions.
Through a series of engaging examples and practice problems, this worksheet unravels the mysteries of molarity and molality, equipping you with the knowledge to confidently navigate the complexities of chemical reactions and solution behavior.
Introduction to Molarity and Molality
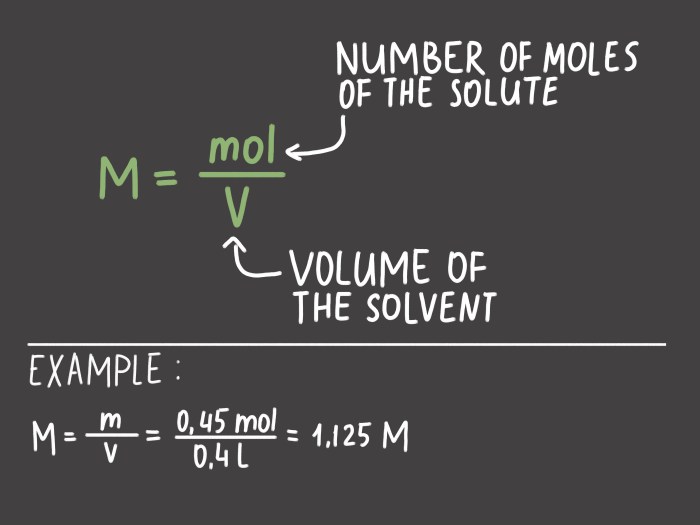
Molarity and molality are two important concentration units used in chemistry. Molarity is defined as the number of moles of solute per liter of solution, while molality is defined as the number of moles of solute per kilogram of solvent.
Molarity is a more commonly used concentration unit than molality, and it is often used in titrations and acid-base reactions. Molality is often used in freezing point depression and boiling point elevation calculations.
Calculations Involving Molarity and Molality, Concentrations worksheet molarity and molality
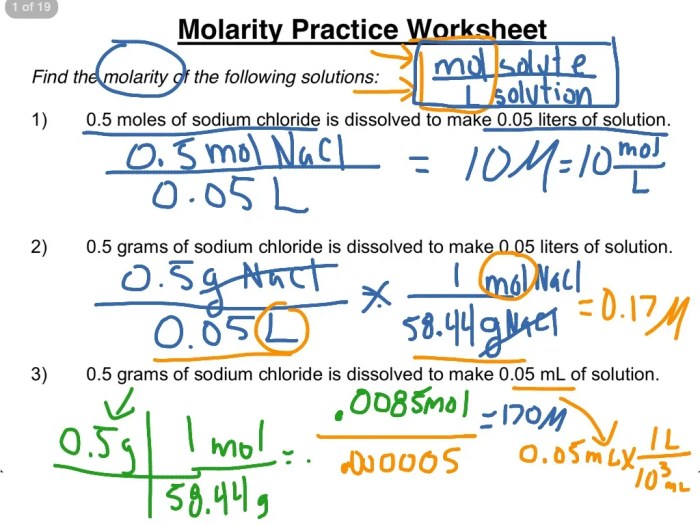
To calculate the molarity of a solution, divide the number of moles of solute by the volume of the solution in liters.
To calculate the molality of a solution, divide the number of moles of solute by the mass of the solvent in kilograms.
Applications of Molarity and Molality
Molarity is used in a variety of chemistry applications, including:
- Titrations
- Acid-base reactions
- Buffer solutions
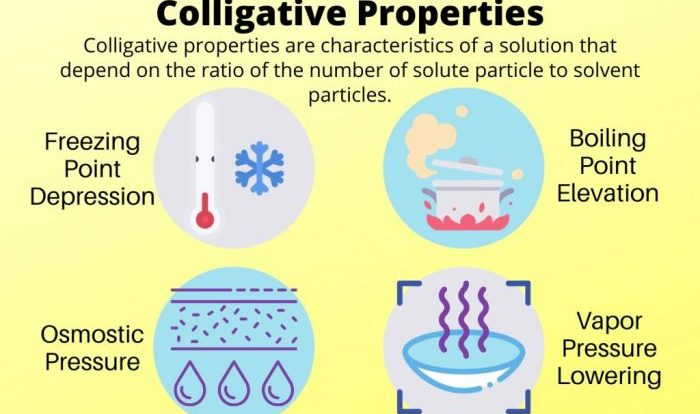
Molality is used in a variety of chemistry applications, including:
- Freezing point depression
- Boiling point elevation
- Osmotic pressure
Key Questions Answered: Concentrations Worksheet Molarity And Molality
What is the difference between molarity and molality?
Molarity measures the number of moles of solute per liter of solution, while molality measures the number of moles of solute per kilogram of solvent.
How do I calculate the molarity of a solution?
Molarity = moles of solute / liters of solution
What are some applications of molarity and molality?
Molarity is used in titrations and acid-base reactions, while molality is used in freezing point depression and boiling point elevation.