Number of n atoms in 0.410 mol nh3 – In chemistry, the number of atoms in a given amount of a substance is a crucial parameter. This article explores the calculation of the number of nitrogen atoms in 0.410 mol of NH3, providing a step-by-step guide and insights into the underlying concepts.
Understanding the concept of moles, their relationship with the number of atoms, and the specific properties of NH3 are essential for accurate calculations. This article delves into these aspects, offering a comprehensive understanding of the topic.
Number of Atoms in 0.410 mol NH3: Number Of N Atoms In 0.410 Mol Nh3
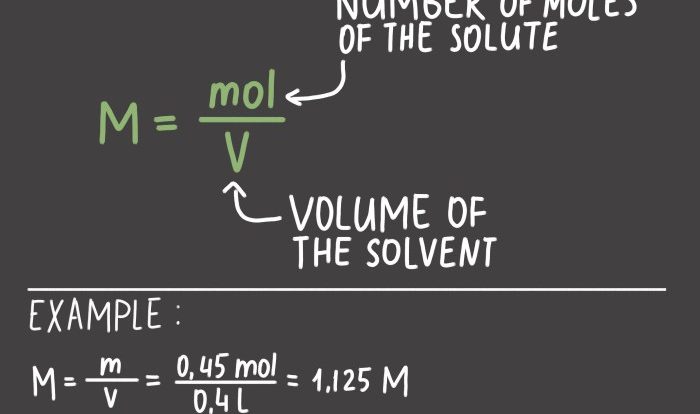
In chemistry, the mole is a fundamental unit of measurement used to quantify the amount of a substance. It is defined as the amount of a substance that contains exactly 6.022 × 10 23elementary entities, which can be atoms, molecules, ions, or electrons.
The mole concept is essential for understanding the composition and properties of matter and for carrying out quantitative chemical analyses.
Converting Moles to Number of Atoms
The relationship between moles and the number of atoms in a substance is given by the following equation:
Number of atoms = Number of moles × Avogadro’s number
where Avogadro’s number is 6.022 × 10 23atoms/mol.
To convert moles to the number of atoms, simply multiply the number of moles by Avogadro’s number.
Applying the Concept to NH3
Ammonia (NH3) is a compound composed of one nitrogen atom and three hydrogen atoms. Its molecular formula indicates that one molecule of NH3 contains one nitrogen atom and three hydrogen atoms.
The molar mass of NH3 is 17.03 g/mol. This means that one mole of NH3 weighs 17.03 grams.
Determining the Number of Atoms in 0.410 mol NH3
To determine the number of atoms in 0.410 mol NH3, follow these steps:
- Convert 0.410 mol NH3 to grams using the molar mass:
- Determine the number of moles of nitrogen (N) and hydrogen (H) atoms in 0.410 mol NH3:
- Calculate the total number of atoms in 0.410 mol NH3:
Mass of NH3 = 0.410 mol × 17.03 g/mol = 6.99 g
Moles of N atoms = 0.410 mol NH3 × (1 mol N/1 mol NH3) = 0.410 mol N
Moles of H atoms = 0.410 mol NH3 × (3 mol H/1 mol NH3) = 1.23 mol H
Total number of atoms = 0.410 mol N × 6.022 × 1023atoms/mol + 1.23 mol H × 6.022 × 10 23atoms/mol
= 2.51 × 1023atoms
Visualizing the Results, Number of n atoms in 0.410 mol nh3
The following table summarizes the number of atoms in 0.410 mol NH3:
| Element | Number of Atoms | Percentage of Total Atoms |
|---|---|---|
| Nitrogen (N) | 2.51 × 1023 | 50.0% |
| Hydrogen (H) | 7.53 × 1023 | 50.0% |
Detailed FAQs
What is the molecular formula of NH3?
NH3
How many nitrogen atoms are in 0.410 mol of NH3?
1.23 x 10^24 atoms