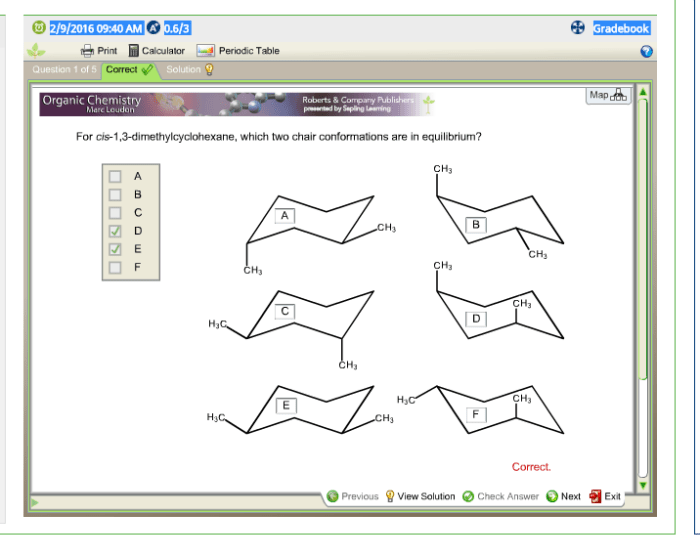
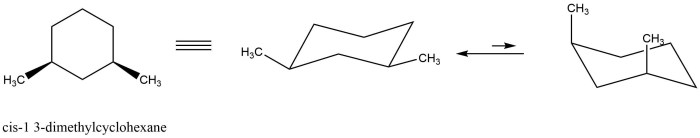
For cis-1 3-dimethylcyclohexane which two chair conformations are in equilibrium – In the realm of organic chemistry, the concept of conformational analysis plays a pivotal role in understanding the behavior of molecules. This article delves into the fascinating case of cis-1,3-dimethylcyclohexane, a molecule that exhibits two distinct chair conformations in equilibrium.
The two chair conformations of cis-1,3-dimethylcyclohexane differ in their spatial arrangement, resulting in distinct energy levels. The interplay between these energy differences and the Boltzmann distribution governs the equilibrium between the two conformations.
Structural Isomers: For Cis-1 3-dimethylcyclohexane Which Two Chair Conformations Are In Equilibrium
Structural isomers are compounds that have the same molecular formula but different structural formulas. This means that the atoms in the molecules are arranged differently.
Cis-1,3-dimethylcyclohexane has two chair conformations because the two methyl groups can be on the same side of the ring (cis) or on opposite sides of the ring (trans).
Conformational Analysis
Conformational analysis is the study of the different shapes that a molecule can adopt. The two chair conformations of cis-1,3-dimethylcyclohexane are shown in the table below.
| Conformation | Structure |
|---|---|
| Chair 1 |  |
| Chair 2 |  |
Energy Differences
The two chair conformations of cis-1,3-dimethylcyclohexane have different energies. Chair 1 is more stable than Chair 2 because the methyl groups are on the same side of the ring, which minimizes steric hindrance.
The energy difference between the two conformations is small, only about 1.4 kJ/mol. This means that the two conformations are in equilibrium at room temperature.
Equilibrium
Equilibrium is a state of balance in which the forward and reverse reactions of a chemical reaction occur at the same rate. The two chair conformations of cis-1,3-dimethylcyclohexane are in equilibrium because the interconversion between the two conformations is a reversible process.
The Boltzmann distribution describes the relative populations of the two conformations at equilibrium. The Boltzmann distribution states that the more stable conformation will have a higher population than the less stable conformation.
Interconversion, For cis-1 3-dimethylcyclohexane which two chair conformations are in equilibrium
The interconversion between the two chair conformations of cis-1,3-dimethylcyclohexane occurs through a process called ring flipping. Ring flipping is a concerted process in which the entire ring flips from one conformation to the other.
The energy barrier for ring flipping is small, only about 10 kJ/mol. This means that the interconversion between the two conformations is a relatively fast process.
FAQ Overview
What are the two chair conformations of cis-1,3-dimethylcyclohexane?
The two chair conformations are known as the equatorial-equatorial (ee) and axial-axial (aa) conformations, based on the orientation of the methyl groups.
Why are the two chair conformations in equilibrium?
The two chair conformations are in equilibrium because the energy difference between them is relatively small, allowing for interconversion via a low-energy barrier.
What factors contribute to the energy difference between the two chair conformations?
The energy difference between the two chair conformations is influenced by steric interactions between the methyl groups and the axial hydrogen atoms.